Webinar on Policies for Supervision of Nuclear Medicine Facilities and the Development of Latest Nuclear Medicine Modalities and Services
Kembali 09 September 2020 | Berita BAPETEN | 1111 lihatIn order to increase knowledge and insight related to the utilization of ionizing radiation sources in the field of nuclear medicine, BAPETEN held a "Webinar on Policies for Supervision of Nuclear Medical Facilities and the Development of Latest Nuclear Medicine Modalities and Services."
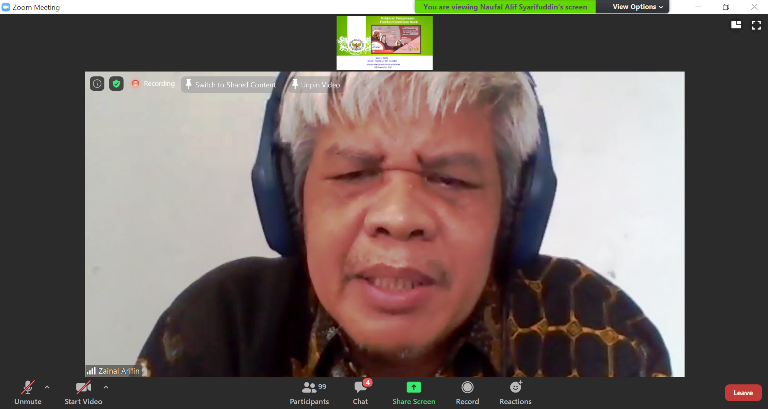
The event was opened by the Deputy Chairman for Licensing and Inspection of BAPETEN Ir. Zainal Arifin, MT. In his remarks, he convey, "This is the 2nd Webinar, previously with the Chairman of the Indonesian Radiation Oncology Society (PORI) Prof. Dr. dr. Soehartati Gondhowiardjo. This event was held in order to understand the aspects of nuclear energy utilization in the medical field. Its developments include, such as Inuki, who was originally engaged in the industrial sector, now turning into a pharmaceutical company. Participants are expected to be able to hold discussions about the latest nuclear developments."
"BAPETEN will encourage the Directorate for Licensing and the Directorate for Inspection so that applying for permits are not difficult and the validity period of the permit can also be longer, according to the amandement of PP 29 concerning Licensing for Ionizing Radiation Sources, which hopefully can be completed soon," he added.
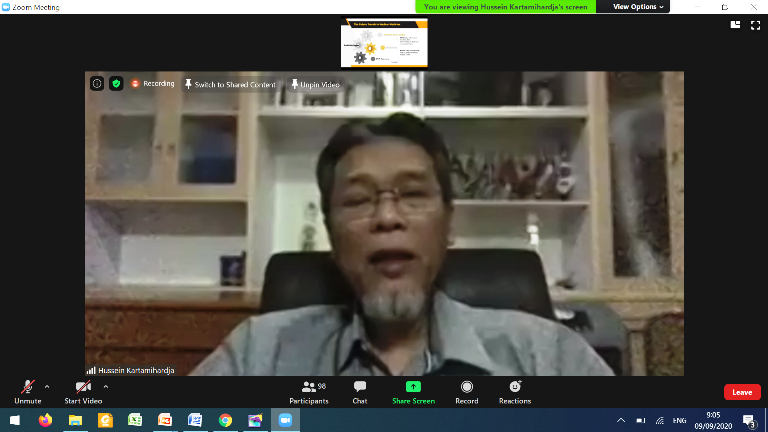
The event was continued with presentation on "Nuclear Medical Facility Supervision Policy" by the Deputy Chairman for Licensing and Inspection . And continued with "Development of Latest Nuclear Medicine Modalities and Services, National and International" by Dr. dr. Achmad Hussein Sundawa Kartamihardja SpKN-TM (K)., MH. Kes., FANMB. as a Specialist Doctor in Nuclear Medicine of Hasan Sadikin General Hospital/Professor of the Department of Nuclear Medicine and Molecular Imaging, Faculty of Medicine of Padjajaran University.
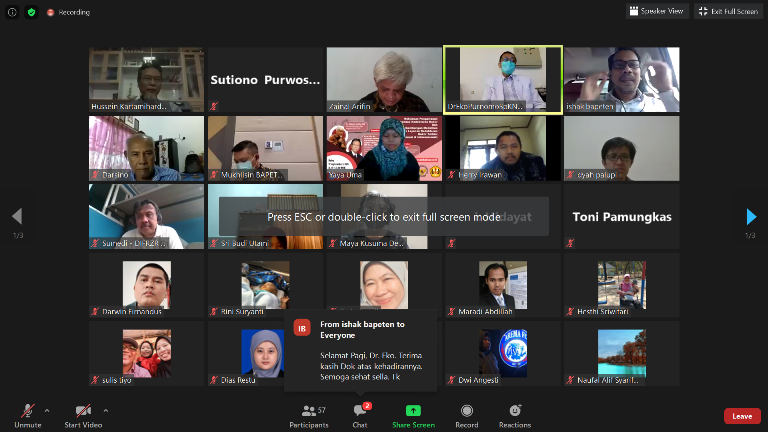
Webinar who was moderated by the Director for Licensing of Radiation Facilities and Radioactive Substances of BAPETEN Ishak, M.Si., held on Wednesday (09/09) and attended by 100 participants from BAPETEN, Batan, and several hospital representatives.
In the question and answer session via chat that read by the moderator, there were many questions from the participants, including the direction of policy between regulators such as BAPETEN, Ministry of Health, and National Agency of Drug and Food Control of Republic of Indonesia (BPOM) related to radiopharmaceuticals, the development of radiopharmaceuticals abroad, and how the future plans of national policy makers. [BHKK/SP]
Presentations can be downloaded: https://drive.google.com/drive/folders/1VS4p18_leb
1. Supervision Policy click here